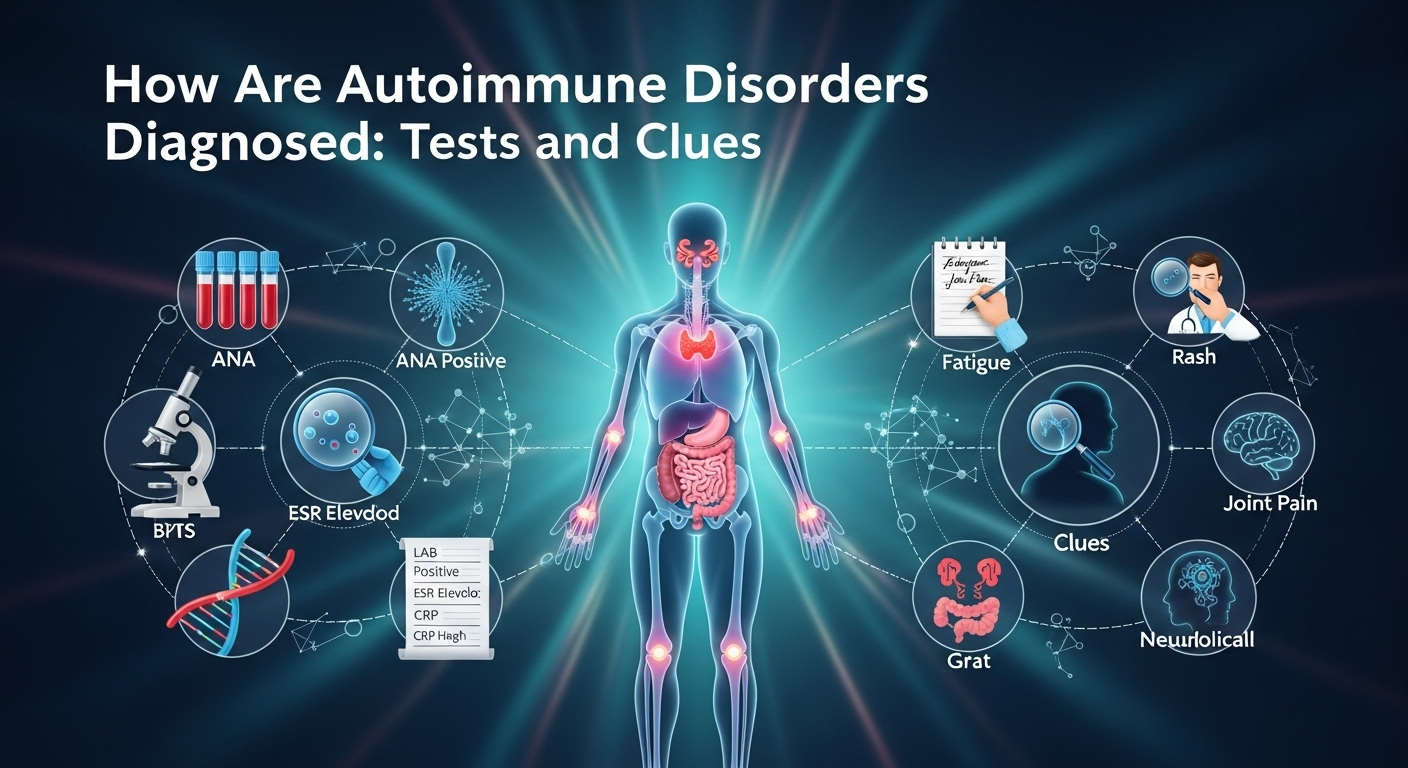
How Are Autoimmune Disorders Diagnosed: Tests and Clues When your immune system starts attacking your own tissues, symptoms can look vague, overlapping, or even contradictory. That’s why the question many people ask—how are autoimmune disorders diagnosed—doesn’t have a one-size-fits-all answer. Instead, clinicians combine patterns from your story, exam clues, targeted labs, imaging, and sometimes biopsies. This guide walks you through that process in plain language, so you can understand the logic behind each test, what “positive” actually means, and how doctors connect the dots to arrive at a confident diagnosis. Understanding Autoimmune Disorders and Why Diagnosis Is Tricky Autoimmune disorders share a common theme: immune misrecognition of self. But they do not share a single blueprint. Lupus can affect skin, joints, kidneys, brain, and blood; autoimmune thyroid disease may focus on the thyroid; celiac disease targets the small intestine; multiple sclerosis affects the central nervous system. The result is a spectrum of presentations, which can shift over time. Early in disease, you may have only fatigue and joint stiffness or rashes that come and go—clues that are easy to miss. Complicating matters, many autoimmune tests are not black-and-white. An antinuclear antibody (ANA) test may be positive in healthy individuals, especially at low titers, while some people with bona fide autoimmune disease may test negative early on. These false positives and false negatives are why doctors never rely on a single test. Instead, they weigh probabilities, track patterns across visits, and use follow-up testing to refine the picture. Diagnosis is also a process of exclusion. Infections, malignancies, and certain medications can mimic autoimmunity or even trigger it de novo. Because treatments for autoimmune disease often suppress the immune system, ruling out other causes first is critical. The bottom line: a correct diagnosis blends clinical judgment with targeted testing—not testing alone. The Stepwise Diagnostic Approach Clinicians Use The best clinicians use a stepwise algorithm that starts broad and narrows with each clue. That approach minimizes unnecessary tests and focuses on pretest probability—the chance you have a disease before testing. When the pretest probability is considered, test results are more meaningful. Your journey often starts with a primary care clinician who orders baseline labs and looks for red flags. If an autoimmune disease is suspected, you may be referred to a rheumatologist, neurologist, gastroenterologist, endocrinologist, or dermatologist depending on which organs are mainly involved. Early, focused referrals help prevent diagnostic delay and reduce complications. Over time, the picture may evolve. Symptoms can declare themselves more clearly, a rash might appear, or a biomarker may change. That’s why longitudinal data—notes, labs, imaging over months—is invaluable. It reveals trends and correlations that a single snapshot can’t. 1) History and Symptom Pattern Recognition Doctors start with your story: when symptoms began, their sequence, triggers, and what helps or worsens them. Patterns matter. For example, morning stiffness lasting more than 30–60 minutes points toward inflammatory arthritis, while fatigue and an itchy rash after gluten exposure may flag celiac disease. They also ask about family history, prior infections, and medications. Some drugs can induce lupus-like syndromes or cause a positive ANA. Travel history might suggest infections that mimic autoimmune disease. Symptom clusters—dry eyes and mouth with parotid swelling, photosensitive rashes with joint pain, or relapsing neurological deficits—helps narrow the field. Doctors will probe systemic features: fevers, weight changes, mouth ulcers, Raynaud’s phenomenon, rashes, chest pain, shortness of breath, abdominal pain, neurological symptoms, and urinary changes. Each adds or subtracts probability for specific diseases. The history is the clinician’s first and most sensitive test. 2) Physical Examination: Clues on Skin, Joints, and Organs A careful exam can be remarkably revealing. A malar “butterfly” rash suggests lupus, while silvery plaques over extensor surfaces point toward psoriasis. Nail pitting or onycholysis reinforces psoriatic arthritis suspicion. Livedo reticularis may suggest vasculitis or antiphospholipid syndrome. Joint exams look for swelling, warmth, and tenderness with symmetrical patterns hinting at rheumatoid arthritis. Muscle strength testing can reveal proximal weakness characteristic of inflammatory myopathies. Checking glands, lymph nodes, and abdominal organs may uncover enlargement or tenderness that suggests systemic involvement. Cardiopulmonary exams can reveal pleurisy, pericardial rubs, or rales suggesting interstitial lung disease. Neurologic exams assess reflexes, sensation, and cranial nerves—key in multiple sclerosis or peripheral neuropathies. These bedside clues guide which tests to order next and prevent shotgun testing. 3) Baseline Labs: CBC, CMP, and Inflammation Markers Initial lab work typically includes a complete blood count (CBC), comprehensive metabolic panel (CMP), and markers of inflammation like erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Anemia, leukopenia, or thrombocytopenia can occur in lupus or as medication side effects. Elevated liver enzymes may reflect autoimmune liver disease, drug-induced injury, or viral hepatitis. ESR and CRP are non-specific but helpful for tracking trends. Persistently high CRP suggests ongoing inflammation; however, some autoimmune diseases (like lupus) may have high ESR with normal CRP. Kidney function and urinalysis can reveal proteinuria or hematuria suggestive of glomerulonephritis, prompting further testing or biopsy. From these basics, clinicians decide whether to proceed to autoantibody panels, imaging, or organ-specific tests. Thoughtful sequencing avoids unnecessary costs and incidental findings that can muddy the waters. Key Blood Tests and What They Mean Blood tests are powerful but must be interpreted in context. A “positive” result increases suspicion only if pretest probability is reasonable and the pattern fits. Conversely, a negative test doesn’t always rule out disease—especially early in the course. A common starting point is the ANA test, which screens for antinuclear antibodies. If ANA is positive and symptoms align, doctors may order more specific extended panels (ENA) to pinpoint likely diagnoses. Disease-specific autoantibodies can dramatically elevate certainty when they align with clinical features. It’s crucial to understand that labs differ in testing methodologies (ELISA, immunofluorescence, chemiluminescence), reference ranges, and cutoffs. Always interpret results using your lab’s standards and in conversation with your clinician. 1) ANA and ENA Panels: Interpreting Titers and Patterns An ANA is reported as a titer (e.g., 1:80, 1:160, 1:320) and a staining pattern (homogeneous, speckled, nucleolar, centromere). Higher titers generally
Charlie Kirk on DNA claims after Colorado school shooting
Charlie Kirk on DNA claims after Colorado school shooting Public conversation after a tragedy is often raw, fast, and polarized. In the hours following reports of a Colorado school shooting, social media feeds filled with posts, hot takes, and speculation—including claims about genetics. This article examines charlie kirk, dna, colorado school shooting claims that circulated online. It explains what’s verified, what isn’t, what the science of genetics can and cannot say, and how to separate rhetoric from evidence when emotions and algorithms run high. By taking a careful, evidence-centered approach, the goal here is not to score political points but to help readers understand how high-profile commentary works, why references to “DNA” enter the conversation after violent events, and what responsible discourse looks like when the facts are still unfolding. As always, accountability and empathy must sit alongside accuracy and context. Finally, because searches spike for key terms after breaking news, this piece is structured for search intent and long-term relevance: it answers FAQs, provides a clear science primer, and maps a repeatable method for verifying claims around any public figure or commentator—not only Charlie Kirk. H2: Context, claims, and why the story spreadH3: 1) What happened, and how online narratives formIn the wake of a Colorado school shooting, platforms prioritize recency and engagement. Posts that contain provocative framings—especially ones that imply a simple cause—tend to spread faster than sober, sourced updates. That’s the core tension: people crave immediate explanation before investigators have released police briefings, timelines, or motives. Into that vacuum, references to “DNA” sometimes surface. These can range from vague insinuations (“there’s something in the DNA of this generation”) to more direct but still nonspecific claims about biology, ancestry, or identity. The allure is understandable: biological explanations feel definitive, and the acronym “DNA” carries scientific gravitas even when used loosely. It’s important to note that specific quotes attributed to any public figure after a chaotic event may be incomplete, decontextualized, or incorrect. Screenshots circulate without timestamps. Edits remove qualifiers. Until a full video, transcript, or verified post is available, treat early virality as a signal to pause, not to conclude. H3: 2) Charlie Kirk’s platform and why his words travelCharlie Kirk is a conservative commentator and the founder of Turning Point USA. With a large audience across radio, podcasts, and social media, his commentary often frames cultural and political debates for millions. That scale matters for two reasons: first, a single phrase can become a headline; second, paraphrases of his remarks—accurate or not—can travel faster than source material. Because of that influence, fact-checkers, journalists, and critics frequently scrutinize what he says, and supporters amplify it. This attention ecosystem means anything connected to Kirk’s name will index quickly in search, get embedded in posts, and potentially anchor public perception of an event even before official facts are known. Even when specific “DNA” quotes are ambiguous or disputed, the conversation around them can steer public debate—prompting questions about genetics, responsibility, and policy. That’s why precise sourcing is crucial and why this article emphasizes verification over virality. H3: 3) What’s verified vs. what’s speculatedAs of publication, public reporting shows that claims linking a Colorado school shooter’s behavior to “DNA” are largely framed by social posts and commentary, not by peer-reviewed evidence. When particular statements are pinned to a named person, the standard is a clear, full-length primary source: a public speech, full podcast episode, or official post. Without that, what remains are interpretations and inferences. Some online accounts may attribute language to a figure like Charlie Kirk that doesn’t appear in a full recording. Others may extrapolate from broader cultural commentary to imply a genetic claim. Until a primary source is produced, those attributions should be treated as unverified. H2: The “DNA” frame: why it resonates and why it misleadsH3: 1) How the “DNA” meme repeats after tragedies“DNA” claims surface after mass violence because they promise a root cause—something inborn, fixed, and explanatory. The meme-like structure repeats across incidents: a tragedy occurs, a viral post references “DNA” or “genetics,” and a debate ensues about whether the speaker blamed biology for social outcomes. It’s a template, and templates drive engagement. There’s also a rhetorical edge: invoking DNA can serve as a proxy for arguments about culture, identity, or morality without naming them directly. That vagueness makes the claim harder to falsify and easier to retweet. It also creates room for confirmation bias; audiences project their beliefs onto an elastic phrase. The problem is that elasticity obscures details. Did the speaker mean forensic DNA at a crime scene? Population genetics? Behavioral predisposition? A metaphor for “deeply rooted”? Without clarity, one acronym stands in for multiple ideas that do not have the same evidentiary basis. H3: 2) Why genetic shorthand is powerful—but can be harmfulThe cultural power of DNA stems from its status as the blueprint of life. Yet genetic determinism—the idea that genes straightforwardly cause complex behaviors—is not supported by current science. Environment, development, social networks, trauma, access to support, and a host of non-genetic factors intertwine with genetic predispositions. When the public conflates DNA with destiny, it risks stigmatizing groups, legitimizing discrimination, or diverting attention from actionable interventions. After a Colorado school shooting, using “DNA” as a catch-all risks muddying the policy conversation: instead of focusing on evidence-backed steps (threat assessment, safe storage practices, mental health services), the debate shifts to abstractions. In short, the “DNA” frame resonates because it’s simple. It misleads because violence is not. H2: Science check: what genetics can and cannot tell us about violenceH3: 1) Behavioral genetics 101Behavioral genetics studies how genetic variation correlates with behavior across populations. Findings often report heritability estimates for traits like impulsivity or risk-taking—but heritability is a population statistic, not an individual verdict. A trait can be partly heritable and still be profoundly shaped by context. For complex behaviors like criminal violence, single-gene explanations are not credible. Instead, thousands of variants, each exerting tiny effects, interact with environments across development. Even then, such statistical associations do not predict individual actions. No